Helix BioPharma Corp. - New directions in cervical cancer prevention
.jpg)
ADVOCACY AWARENESS
Helix BioPharma welcomes the opportunity to raise awareness about its Topical Interferon Alpha-2b program for low-grade cervical lesions among advocates dedicated to the prevention and elimination of cervical cancer.
NEW DIRECTIONS IN CERVICAL CANCER PREVENTION
Helix BioPharma Corp. (“Helix”) is an Ontario-based, clinical-stage biopharmaceutical company developing innovative products for the treatment of cancer and precancerous diseases based on the Company’s proprietary technologies.
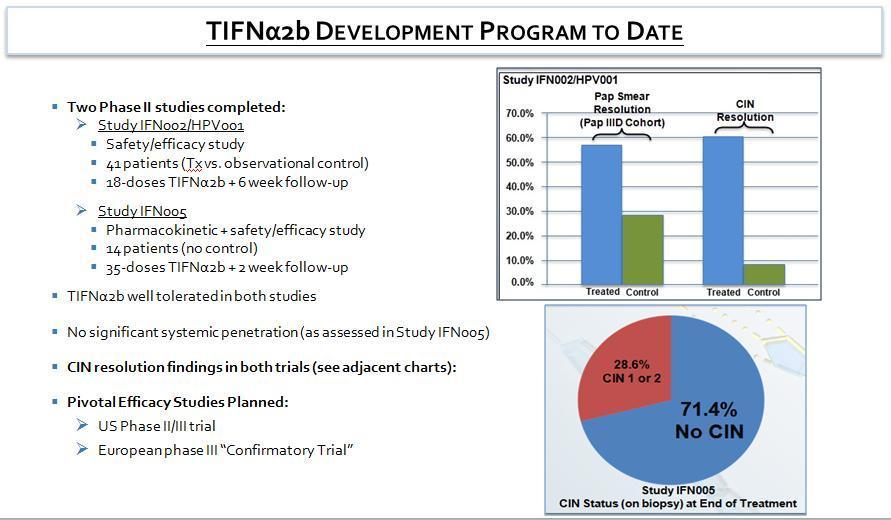
Helix is developing the Company’s late-stage product candidate, Topical Interferon Alpha-2b (“TIFNα2b” Program), which offers a new approach for treating potentially precancerous low-grade cervical lesions caused by the sexually transmitted infection Human Papillomavirus (HPV). Nearly all cases (99.7%) of cervical cancer are associated with the presence of HPV .¹
The TIFNα2b formulation incorporates Helix’s patented Biphasix™ technology, designed to facilitate the topical delivery of macromolecules, such as Interferon Alpha-2b, into the cervical epidermis. TIFNα2b is intended to combat HPV infections that otherwise would potentially cause abnormal cellular proliferation in the form of cervical lesions.
An application for a US Phase II/III IND trial for low-grade cervical lesions is currently in progress.
Biphasix™ Technology
Helix’s Drug Candidate: Topical Interferon Alpha-2b (TIFNα2b)
Interferon Alpha-2b therapy, which incorporates Helix’s Biphasix™ technology, is a topical preparation intended for delivering Interferon Alpha-2b drug therapy into the skin and mucosal tissues in HPV infected cervical epidermis. The treatment is intended for self-administered application.
Existing therapies for low-grade cervical lesions including ablative/excisional surgical techniques are painful and can result in scarring and other harmful side effects complicating fertility and pregnancy. A further drawback to current management practices is the emotional distress caused by potentially lengthy pre-treatment observation periods.
Helix’s Biphasix™ technology uses microscopic, biphasic microvesicles formulated as a special self-administered cream, to encapsulate, stabilize, and deliver TIFNα2b into the cervical epidermis. Helix’s therapy is intended to offer maximum coverage of the HPV-infected area widely and effectively.
CERVICAL CANCER PREVENTION
Cervical cancer is the second most prevalent cancer in women worldwide.² It remains the leading cause of cancer-related deaths for women in the majority of developing countries ³. The disease is in need of a new and novel approach for prevention. Nearly all cases (99.7%) of cervical cancer are associated with the presence of the Human Papillomavirus (HPV),¹ currently the most common sexually transmitted infection.4
The National Cancer Institute estimated in 2009 that 1.25 million women in the US would be diagnosed that year with potentially precancerous cervical lesions upon Pap smear assessment (NCI Cervical Cancer Prevention, 2009).
Cervical HPV infection can cause potentially precancerous lesions detectable upon Pap smear and colposcopic assessment, referred to as squamous intraepithelial lesions (SIL) or cervical intraepithelial neoplasia (CIN) respectively. There is no pharmaceutical treatment available for these cervical lesions today. Today, HPV’s primary prevention method is a one-time vaccination given prior to HPV exposure. Hopefully, vaccine administration combined with early education and awareness efforts, will reduce the number of unnecessary cases. This is particularly important in parts of the world with limited access to proper medical assistance. Helix believes its Topical Interferon Alpha-2b will complement the HPV vaccines by offering a safe therapeutic solution for patients that already have or continue to develop HPV-induced cervical lesions despite vaccination.
Key Design Benefits of Topical Interferon Alpha-2b are Intended to:
-
Offer a potent drug therapy for the treatment of low-grade cervical lesions, a condition where no pharmaceutical treatment exists today.
-
Be self-administered by patients in the convenience of their home.
-
Empower women with a safe and proactive pharmaceutical treatment approach vs. traditional “wait and see” observational management practices.
-
Increase women's chances of obviating the need for treatment by potentially dangerous/damaging, ablative/excisional surgical procedures.
CORPORATE CONTACT
Helix BioPharma Corp.
John Docherty-President & COO
docherty@helixbiopharma.com
305 Industrial Parkway South
Aurora, Ontario, Canada L4G 6X7
Tel: (905) 841-2300/ Fax: (905) 841-2244
www.helixbiopharma.com
ADVOCACY CONTACT
Cooper Global Communications, LLC
Richard Cooper - President
rcooper@cgc-us.com
Jennifer K. Zimmons, Ph.D.- Managing Director
jzimmons@cgc-us.com
410 Park Ave., Suite 420
New York, NY 10022
Tel. 212-317-1400/ Fax 212-317-1184
Disclaimer
Nothing in this document is intended as an offer to sell or a solicitation of an offer to buy securities of Helix BioPharma Corp. (“Helix”). It is a short summary of selected information. More detailed information about Helix and Topical Interferon Alpa-2b, including risk factors, is contained in Helix’s public filings with securities regulatory authorities in Canada and the United States at www.sedar.com and www.sec.gov, respectively. The information contained herein is based on Helix’s knowledge at the time of printing this document. Helix makes no guarantee as to the accuracy or completeness of such information and assumes no responsibility or liability with respect thereto. This summary contains forward-looking statements and information about Helix and its Topical Interferon Alpha-2b drug candidate, including Helix’s planned pharmaceutical development program, clinical trials, and commercialization plans. Certain material factors or assumptions are applied in making forward-looking statements and providing forward-looking information, including, but not limited to, receipt of necessary additional funding, successful clinical trials, strategic partner support and regulatory approvals. Helix’s actual results, performance or achievement could differ materially from those expressed in, or implied by, these forward-looking statements and information due to a number of risks and uncertainties, including without limitation, those contained in Helix’s publicly filed documents at the websites referred to above and that Helix’s assumptions may prove to be incorrect. Forward-looking statements and information are based on the beliefs, assumptions, opinions and expectations of Helix’s management at the time they are made, and Helix does not assume any obligation to update any forward-looking statement or information should those beliefs, assumptions, opinions or expectations change except as required by law.
References
(1.) Ault, K.A. Epidemiology and Natural History of Human Papillomavirus Infections in the Female Genital Tract. Infec Dis Obs Gyn; 2006; 1-5.
(2.) National Cervical Cancer Coalition (NCCC) Available at: www.nccc-online.org - Accessed March 16,2011
The World Health Organization (WHO) Available at: www.who.int/entity/mediacentre/factsheets/fs297/en/index.html - Accessed March 16,2011
(3.) Ferlay J, Bray P, Pizani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, mortality and prevalence worldwide. IARC CancerBase No 5, Version 2 0 IARCPress, Lyon, 2004. Available at: http://www.iarc.fr - Accessed September 20, 2005
(4.) Insinga R, Glass A, Rush B. The Health Care Costs of Cervical Human Papillomavirus – Related Disease. American Journal of Obstetrics and Gynecology 2004; 191: 114-20.